Live
- Srikakulam: Jagan cautions people against unholy alliance of TDP-BJP-JSP
- Gukesh’s tactical brilliance stuns chess world
- BJP releases ‘charge sheet’ against BJD govt
- District Nodal Officer Venkataramana announced the intermediate results
- She team that stopped child marriages
- Medical devices sector needs separate rules, says industry body AiMeD
- Andhra Paper declares lockout
- Opposition INDIA bloc working on 'one year, one PM' formula: PM Modi
- Delhi HC quashes FIR for outraging woman's modesty, orders man to assist Traffic Police for a month as settlement
- 'Apologise to families of farmers who committed suicide in Vidarbha', Amit Shah dares Sharad Pawar
Just In
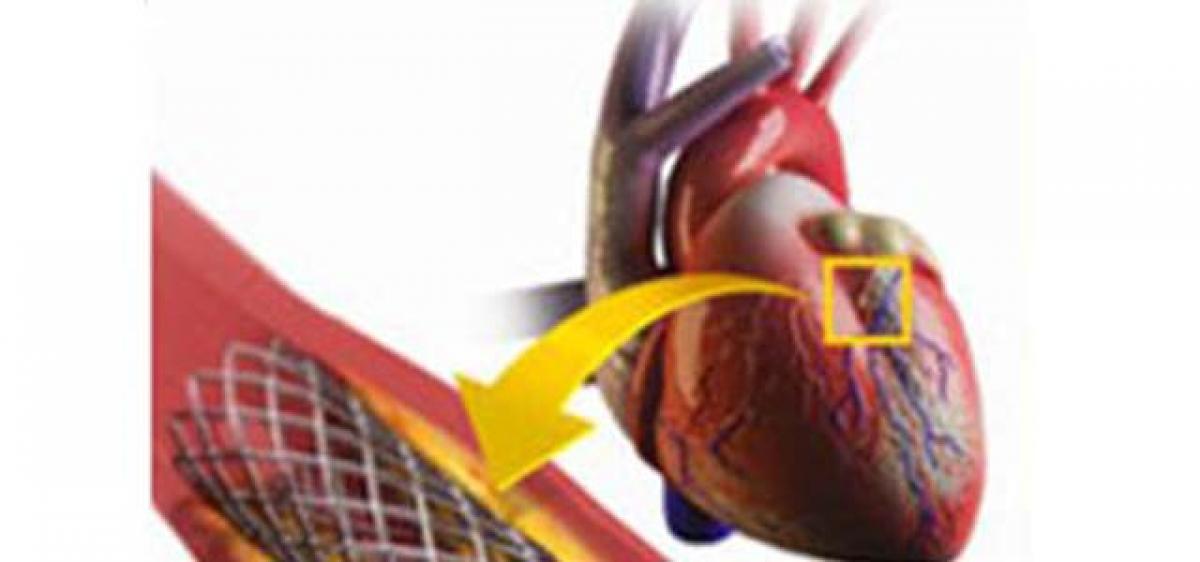
All new generation stents have been withdrawn from the market following the National Pharmaceutical Pricing Authority’s (NPPA’s) order capping prices last week.
Hyderabad: All new generation stents have been withdrawn from the market following the National Pharmaceutical Pricing Authority’s (NPPA’s) order capping prices last week.
Several patients, who wish to go in for high-end stents, are now planning to go abroad and get treatment. While corporate hospitals are tight-lipped on the issue, doctors and people in the know of things on condition of anonymity say that many surgeries are now put on hold.
Cardiologist Sashikant says that the situation could lead to reverse medical tourism. Explaining the present condition with an analogy, he says, the government wants all to use Rs 2 kilo rice when one wishes to buy basmati. It is infringement on the rights of a person.
Companies are withdrawing premium cardiac stents from India using the provision in the Drug Price Control Order 2013 which stipulates that any drug brought under price control can be withdrawn from the market after providing a six-month notice to NPPA.
Authorities at Gandhi Hospital and Osmania Hospital say that there is no shortage of stents but corporate hospitals where the bulk of the high-end stents are used are a jittery lot. Apollo Hospital officials said that they have been following the NPPA guidelines.
According to sources, Abbott may withdraw its Xience Alpine and its dissolving stent, Absorb. It was priced Rs 2 lakh before the price cap. NPPA set a price cap of Rs 7,260 on bare metal stents and Rs 29,600 on drug eluting and biodegradable ones.
In the meantime, the government on Wednesday sent a communiqué to manufacturers and distributors that they would have to send a weekly report of supply of stents. The government reiterated that companies, distributors and hospitals need to comply with its order slashing prices of cardiac stents by over 75 per cent and warned them of penalties in case of violations.
Many hospitals have decided to sell only those stents priced within the ceiling fixed by NPPA. A senior surgeon of a corporate hospital says that patients are not too happy with using the ones on offer. Hospitals do not want to sell any stent priced above Rs 30,000.
Raghav, a resident of Banjara Hills said, “My father is planning to go abroad and get treated as hospitals are unwilling to provide third or fourth generation stents anymore.
Presently, foreign players control 80 per cent of the stent market primarily by outbidding Indian ones through kickbacks to doctors and hospitals.
One of the reasons Indian manufacturers are unable to make an entry into government hospitals is the requirement of approval by the US Food and Drugs Administration (FDA) specified in the tenders issued by most government hospitals.
Though the Union Ministry of Health and Family Welfare wrote to all State governments suggesting the removal of FDA-approval requirement from tenders, it was not a notification.

© 2024 Hyderabad Media House Limited/The Hans India. All rights reserved. Powered by hocalwire.com







