Live
- 2 held, 10 red sanders logs seized
- TTD closes Akasaganga, Papavinasam roads
- High costs, limited results: Drone mist-spraying pilot project faces setback
- Poster war on as parties compete for voter attention
- Many dams, tanks filled as rains batter Tirupati dist
- Mayawati, Akhilesh condemn damage to Constitution replica
- Mann slams Centre over ‘one nation, one election’
- Techie suicide case: Mother-in-law flees Jaunpur home
- State government to Supreme Court: New guidelines on how to apply anti-gangster law in UP
- CM Chandrababu to unveil Vision 2047 document today in Vijayawada, traffic restrictions imposed
Just In
Covaxin: 30-year-old given 1st dose of coronavirus vaccine

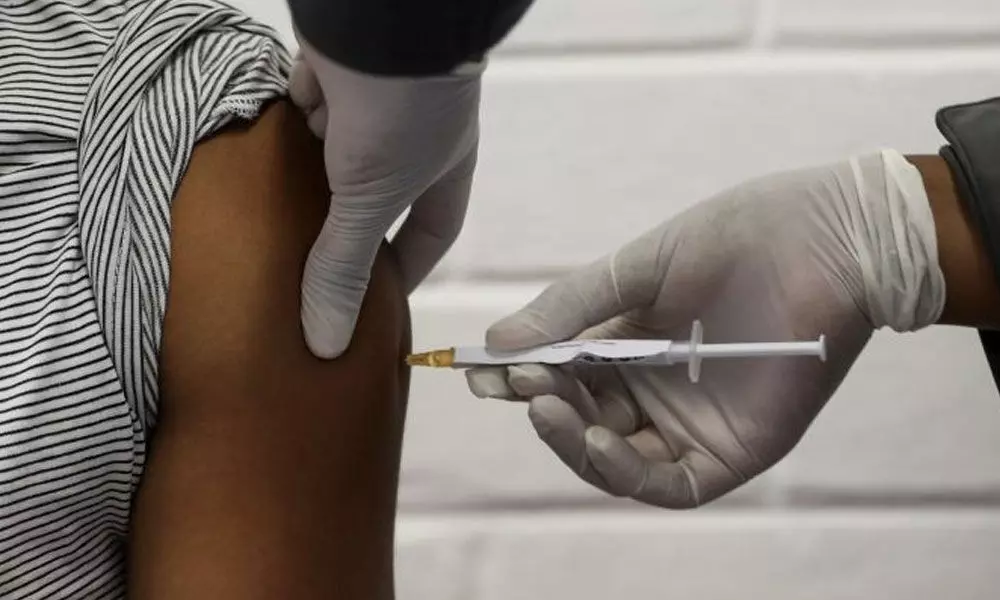
30-yr-old given 1st dose of coronavirus Covaxin
The AIIMS on Friday administered the first dose of country's first indigenous vaccine candidate 'Covaxin' to a 30-year-old man as part of efforts to combat COVID-19.
New Delhi: The All India Institute of Medical Sciences (AIIMS) on Friday administered the first dose of country's first indigenous vaccine candidate 'Covaxin' to a 30-year-old man as part of efforts to combat COVID-19.
This is the first phase of human clinical trial after the institute's ethics committee gave its approval for the trail for the indigenously developed COVID-19 vaccine candidate Covaxin.
Prof (Dr) Sanjay Rai, who works in the Community Medicine Department and is principal investigator of the study, said that dose was administered to a resident of Delhi.
"Today, we administered the first dose of Covaxin to a 30-year-old man, the first volunteer, who is a resident of Delhi. He was screened two days ago and all his health vital parameters were normal. He does not have any co-morbid conditions or any pre-existing illness," Dr Rai told ANI.
"Post-vaccination, the subject was in close observation for two hours. There was no sudden side-effect observed. The subject will be monitored for a week," he said.
Dr Rai said more than 3,500 volunteers have already registered for the trial at AIIMS "Screening for 22 subjects is ongoing and only about a third of them were found to have normal parameters. More volunteers will be vaccinated tomorrow once their health screening reports come," he said.
The patient has been provided with a health card for noting down his daily medical condition and has also been asked to maintain his daily routine. The doctors will check up his health condition daily through telephone for one week post the administration of the vaccine.
According to the Indian Council for Medical Research (ICMR), the trial is taking place at 12 sites for phase I and II randomised, double-blind, placebo-controlled clinical trials of Covaxin. Apart from AIIMS Delhi, trial is taking place at AIIMS Patna and few other sites.
"Any healthy individual who wishes to participate in the trial can send in an email on [email protected] or send an SMS or call on 7428847499," the AIIMS doctor said adding that for phases I & II phase- AIIMS (Delhi) will choose only 100 participants out of 375 volunteers and remaining participants will be studied at other sites.The top drug regulator had earlier given its green signal for human clinical trials for Covaxin, which has been developed by the Hyderabad-based Bharat Biotech in collaboration with the ICMR and the National Institute of Virology (NIV), Pune.

© 2024 Hyderabad Media House Limited/The Hans India. All rights reserved. Powered by hocalwire.com






