Live
- Alia Bhatt captures attention in white
- Varun Dhawan talks about ‘Baby John’
- ‘Moonwalk’ trailer promises a quirky heist, love, and loyalty
- Combat leaf spot disease
- Ahsaas Channaopens up about her complex character in ‘Mismatched 3’
- Radhika Apte welcomes first child, shares heartfelt post
- Jacqueline dazzles at Da-Bangg Reloaded concert
- Time to boost measures to prevent drowning, save children: WHO
- TDP achieves milestone with 73 lakhs membership registration, says Chandrababu
- South Korea: Main Oppn hails Yoon's impeachment motion passage as 'victory for people, democracy'
Just In
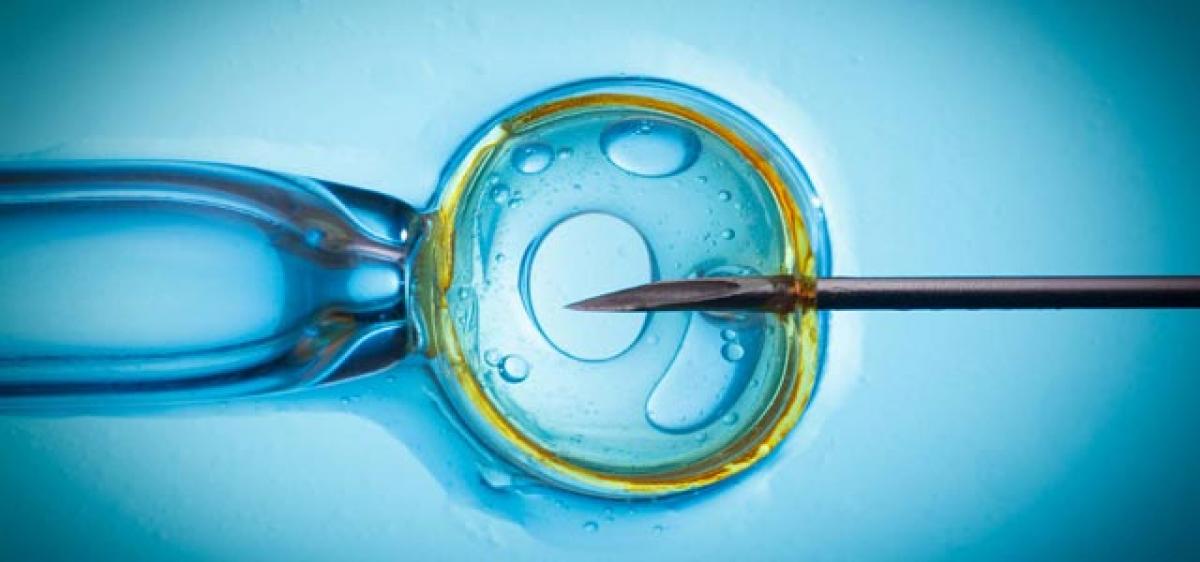
New study results published in Human Reproduction could pave the way for an additional treatment option for the estimated 1.5 million women worldwide who undergo in vitro fertilization (IVF) treatment each year.
New study results published in Human Reproduction could pave the way for an additional treatment option for the estimated 1.5 million women worldwide who undergo in vitro fertilization (IVF) treatment each year.
In the Lotus I study, which involved more than 1,000 women across 38 international sites, oral dydrogesterone had similar efficacy and tolerability to micronized vaginal progesterone (MVP), which is the current standard of care globally to prepare the uterus lining for pregnancy.
IVF is one of several methods of assisted reproduction technology whereby a fertilized embryo is transferred to the woman’s uterus. Progesterone or a related hormone, such as dydrogesterone, is used in IVF to prepare the lining of the uterus (luteal phase support) to allow a fertilized egg to implant.
According to a 2015 analysis by Ernst & Young (EY), infertility has a high prevalence affecting nearly 10 – 15 percent of married couples in India. Nearly 27.5 million couples who are actively seeking children suffer from infertilityvii. Despite the increasing demand for infertility treatment in India, only 1 percent of couples in India seek assisted reproductive treatment such as IVFvii.
MVP is the most commonly used method of administering progesterone in IVF centers globally, but is associated with side effects, such as irritation and discharge, as well as poor patient acceptancevi. Lotus I, a Phase III randomized controlled clinical study, evaluated the effects of oral dydrogesterone in luteal support in IVF.
The findings of the Lotus I study provide clinical evidence for an oral treatment option. Besides its ease of administration, the Lotus I study concluded that oral dydrogesterone is similarly well-tolerated and efficacious compared to MVP.
"The findings from this study have the potential to have important implications for women undergoing IVF," said Herman Tournaye, MD, Ph D, Director of the Center for Reproductive Medicine at Universitair Ziekenhuis Brussel, and lead clinical researcher for the Lotus I study.
"We found oral dydrogesterone to be effective, well tolerated and easy to administer – all of which point to it becoming the new preferred treatment option." In the Lotus I study, oral dydrogesterone achieved similar results to MVP in terms of the presence of fetal heart beats at 12 weeks gestation, representing an ongoing pregnancy. The two treatment groups also had similarpregnancy and live birth rates.
The results from the Lotus I study add to the body of evidence from smaller IVF studies of the benefits of using oral dydrogesterone for luteal support as part of IVF. Abbott manufactures oral dydrogesterone for countries outside of the United States.

© 2024 Hyderabad Media House Limited/The Hans India. All rights reserved. Powered by hocalwire.com







