Johnson & Johnson drops proposal for vaccine approval in India
Johnson & Johnson drops proposal for vaccine approval in India
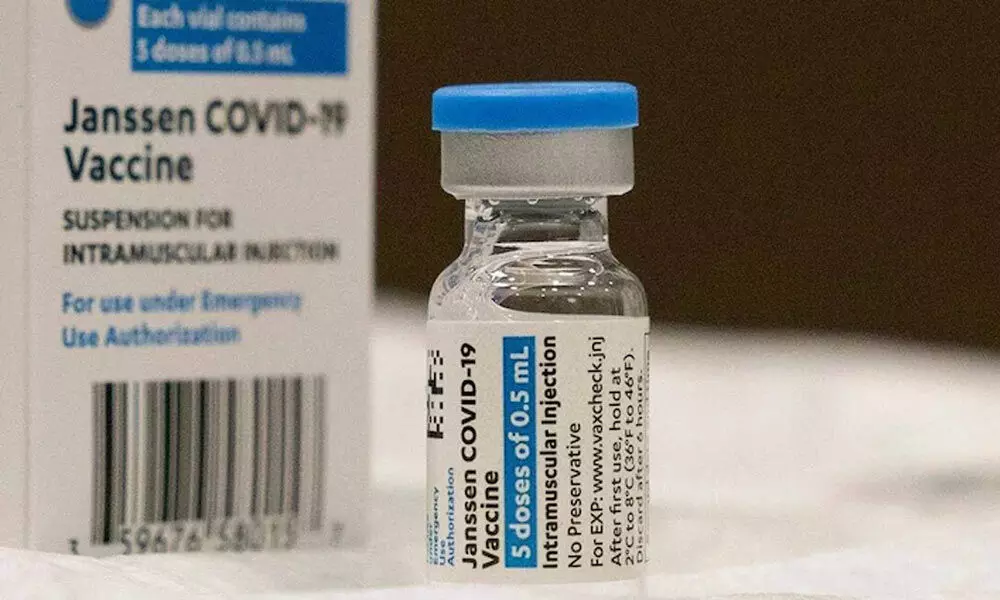
India's drug regulator CDSCO said on Monday that Johnson & Johnson withdrew its proposal seeking accelerated approval of its Covid-19 vaccine in the country, without giving additional details.
New Delhi: India's drug regulator CDSCO said on Monday that Johnson & Johnson withdrew its proposal seeking accelerated approval of its Covid-19 vaccine in the country, without giving additional details.
"The firm has informed that they are withdrawing their proposal," a line in Central Drugs Standard Control Organisation's (CDSCO) note on a meeting held accelerated approval process said.
The meeting was held on July 29. The US-based company had said in April that it was seeking an approval to conduct a bridging clinical study of its Janssen Covid-19 vaccine candidate in India. As per the data available till July 31, Johnson & Johnson is yet to request a full approval for its shot with the US Food and Drug Administration (FDA), while Pfizer Inc, BioNTech SE, and Moderna Inc have already sought full approval of their vaccines with the FDA. Johnson & Johnson has claimed that their vaccine generates a strong and persistent response activity against Delta and other prevalent strains of coronavirus.








