Eye drops slow near-sightedness progression in kids: Study
Eye drops slow near-sightedness progression in kids
The first drug therapy to slow the progression of near-sightedness in children could be on the horizon, suggest results of a new clinical trial
The first drug therapy to slow the progression of near-sightedness in children could be on the horizon, suggest results of a new clinical trial.
About one in three adults worldwide is near-sighted, and the global prevalence of myopia is predicted to increase to 50 per cent by 2050.
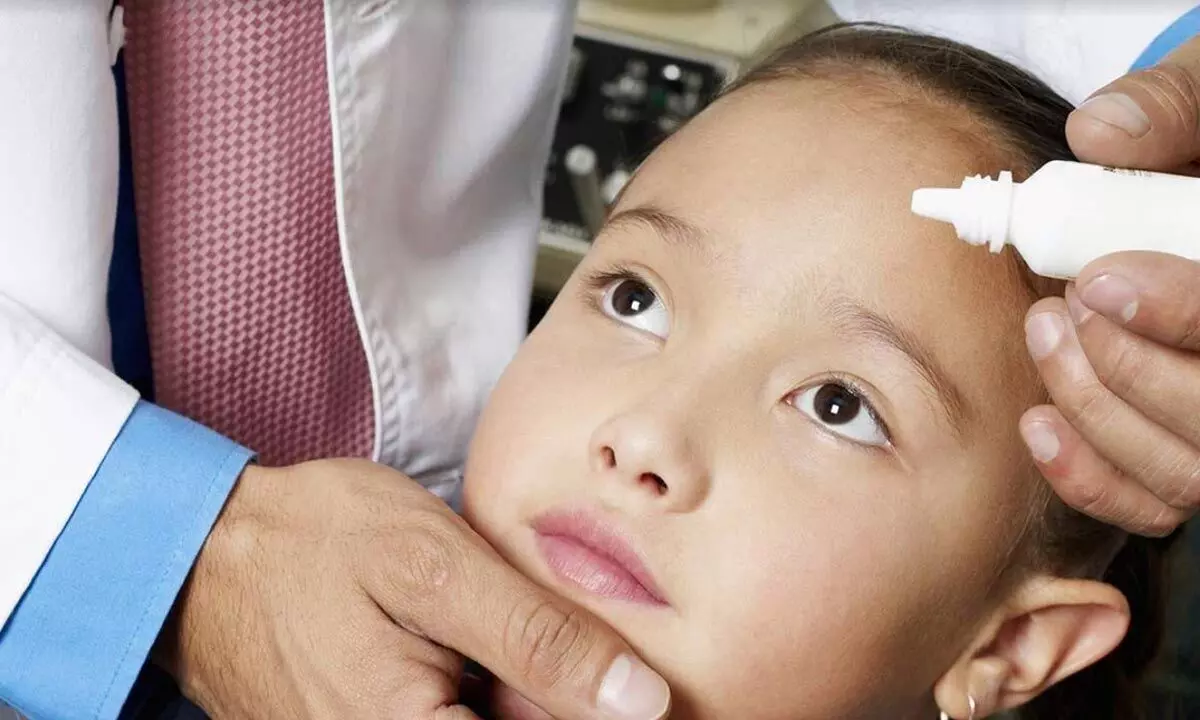
The three-year study found that a daily drop in each eye of a low dose of atropine, a drug used to dilate pupils, was better than a placebo at limiting eyeglass prescription changes and inhibiting elongation of the eye in near-sighted children aged 6 to 10.
That elongation leads to myopia, or near-sightedness, which starts in young kids and continues to get worse into the teen years before levelling off in most people.
In addition to requiring life-long vision correction, near-sightedness increases the risk for retinal detachment, macular degeneration, cataracts and glaucoma later in life -- and most corrective lenses don't do anything to stop myopia progression.
"The idea of keeping eyeballs smaller isn't just so people's glasses are thinner -- it would also be so that in their 70s they don't suffer visual impairment," said lead author Karla Zadnik, Professor and dean of the College of Optometry at The Ohio State University.
Animal studies years ago hinted at atropine's ability to slow the growth of the eye, but the full-strength drug's interference with near vision and concerns about pupil dilation hindered early considerations of its potential as a human therapy for myopia.
More recent research has suggested a low dose of atropine might help.
In the study, published in JAMA Ophthalmology, the team assessed the drug's effectiveness in 489 children aged 6 to 10.
The drugs' safety was assessed in a larger sample of 573 participants that also included children as young as 3 and up to age 16.
The most common side effects were sensitivity to light, allergic conjunctivitis, eye irritation, dilated pupils and blurred vision, although reports of these side effects were few.
The experimental drug is made without preservatives, and if federally approved as a therapy, would be distributed in single-use packaging for convenience and to prevent contamination, the researchers noted.

