Centre blames TN drug regulator for Coldrif tragedy
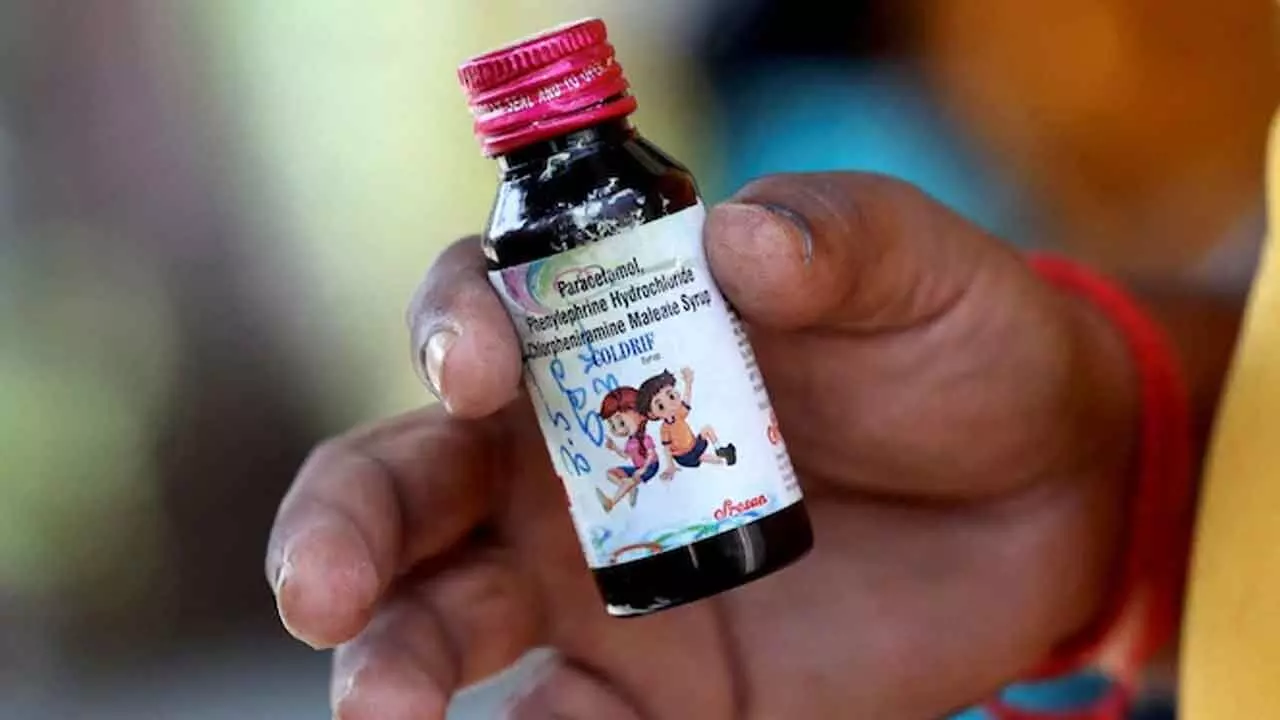
New Delhi: New Delhi: The Centre has squarely blamed the Tamil Nadu Food and Drug Administration (TN-FDA) for gross regulatory lapses in the Coldrif cough syrup tragedy that has claimed the lives of several children across India.
The syrup, manufactured in Tamil Nadu and distributed nationwide, was found to contain diethylene glycol (DEG) — a highly toxic industrial chemical unfit for human consumption. The same batch has been linked to child deaths in Madhya Pradesh and Rajasthan.
A 26-page inspection report by the Tamil Nadu Drugs Control Department revealed more than 350 violations at Sresan Pharmaceuticals, the firm behind Coldrif.
Investigators found unhygienic conditions, rusted machinery, and the illegal use of non-pharma-grade chemicals. The facility reportedly operated without a Quality Assurance department or any standard operating procedures for recalling products. Officials from the Union Ministry of Health and Family Welfare told News9 that the responsibility for enforcing compliance lies with State Drug Controllers, who issue and monitor manufacturing licences.
The Central Drugs Standard Control Organisation (CDSCO) has now recommended the cancellation of Sresan Pharmaceuticals’ licence, citing “persistent neglect” and regulatory failure. However, the final decision rests with the Tamil Nadu drug authority, which has since sealed the plant and issued a show-cause notice to the company.
A Special Investigation Team (SIT) has been formed to probe the deaths, while the company’s owner, Ranganathan Govindarajan, remains absconding.














