Live
- Dhankhar a govt spokesperson, biggest RS disruptor: Kharge
- Fire breaks out at petrol pump
- Facial recognition-based attendance in Sectt from today
- TGPSC to be restructured on par with UPSC
- Gabba pitch to have pace and bounce
- Not taking a break after Olympics left me emotionally drained
- SSC public exams from March 17 to 31
- Pathetic state of Zoo Park-Aramghar underpass turning deadly for commuters
- Losses galore for PCB if it pulls out of Champions Trophy over hosting deadlock
- Govt to support plot owners in build homes
Just In
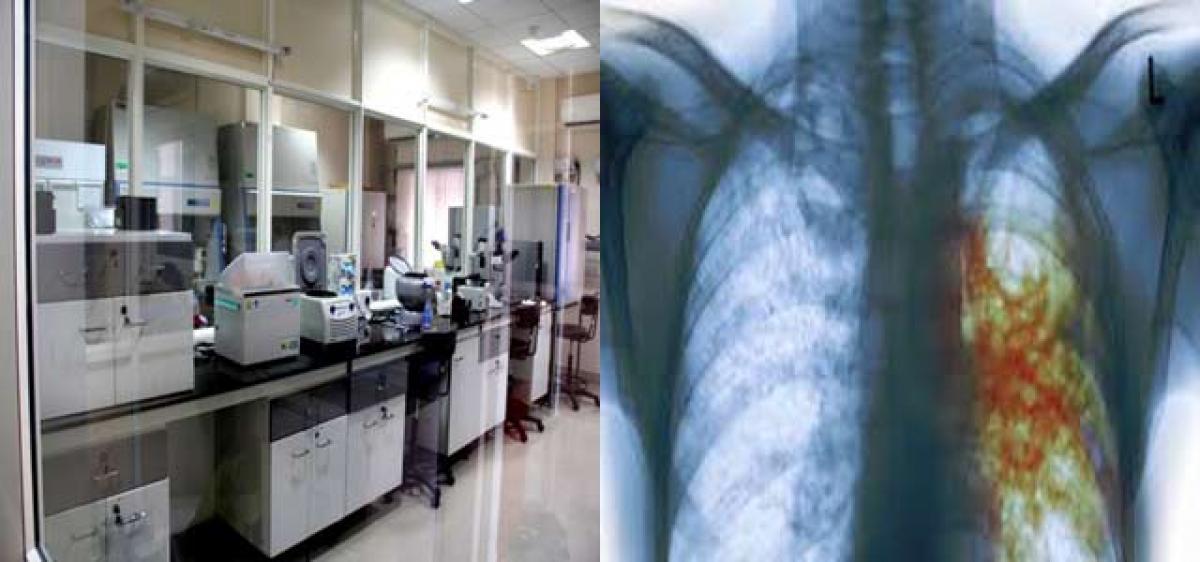
Monetary inability of the patients to afford treatment has resulted in diseases persisting for prolonged periods and at times aggravating to chronic and lethal proportions.
Hyderabad: For a majority of Indians, getting access to efficacious and effective medication for serious and severe ailments has always proved to be beyond their reach due to the high price tags attached to essential drugs.
Monetary inability of the patients to afford treatment has resulted in diseases persisting for prolonged periods and at times aggravating to chronic and lethal proportions.
In a breakthrough that could infuse hope and cure for those afflicted with tuberculosis, Hyderabad-based CSIR-Indian Institute of Chemical Technology (CSIR-IICT) has undertaken the process development of a new drug, that could be the answer not just in terms of affordability, but also in breaking the barriers of resistance in the bacteria that inflicts the disease.
This is a crucial effort in Indian context, considering the fact that till date this is the only drug which has the potency of decimating the multi-drug-resistant (MDR) tuberculosis bacilli.
The drug, Bedaquiline, which acts against drug resistant TB bacillus was developed by Jansen (J&J), USA. CSIR-IICT was funded under the CSIR initiative on the Fast Track Translational (FTT) Projects to develop affordable drugs.
The project led by Dr. S. Chandrasekhar, Director, CSIR-IICT and his team, have taken up the assignment with an objective of developing alternative routes for the production of the TB drug to make the medicine available at an affordable price.
This will not only benefit the Indian patients, but also help those in African and other countries afflicted with TB. In an exclusive interaction with The Hans India, Dr. G. V. M. Sharma, Chief Scientist, and his senior colleague Dr. Prathama Mainkar, Sr. Principal Scientist, explained that the treatment of Tuberculosis has become a challenge because of the disease causing pathogen assuming multi-drug-resistant (MDR) character in the patients, consequently resulting in most of the drugs used in its treatment becoming ineffective and redundant.
Dr. Sharma informed that the TB drug under development by the CSIR-IICT team has been approved by the United States Food and Drug Administration (USFDA) – one of the premier global drug certification organizations.
“Presently, after 60 years, there is only one drug approved by FDA for TB treatment, that has the capability or potency to break through these defenses, but it is too costly and unaffordable for a majority of those suffering from Tuberculosis the world over.”
According to Dr.Sharma, CSIR-IICT will approach the identified Pharma industry for the demonstration and commercialization after completion of the lab scale process. Elucidating on how the Institute will be able to produce the drug at a cheaper price, Dr. Sharma informed that production costs could be decreased through cutting down on the number of operations, modifying the reagents and simplifying the protocols.
He also informed that in addition to the TB drug, CSIR-IICT is working on two more drugs under the FTT initiative, wherein, the processes have to be completed in 12-18 months time. According to the World Health Organisation (WHO) India has over 28 lakh TB patients, with around 64,000 of the cases suspected to be multi-drug-resistant (MDR).
By: Satyapal Menon

© 2024 Hyderabad Media House Limited/The Hans India. All rights reserved. Powered by hocalwire.com







