Alternative to cruel animal testing
We won’t always have to use animals for medical research. Here’s what we can do instead
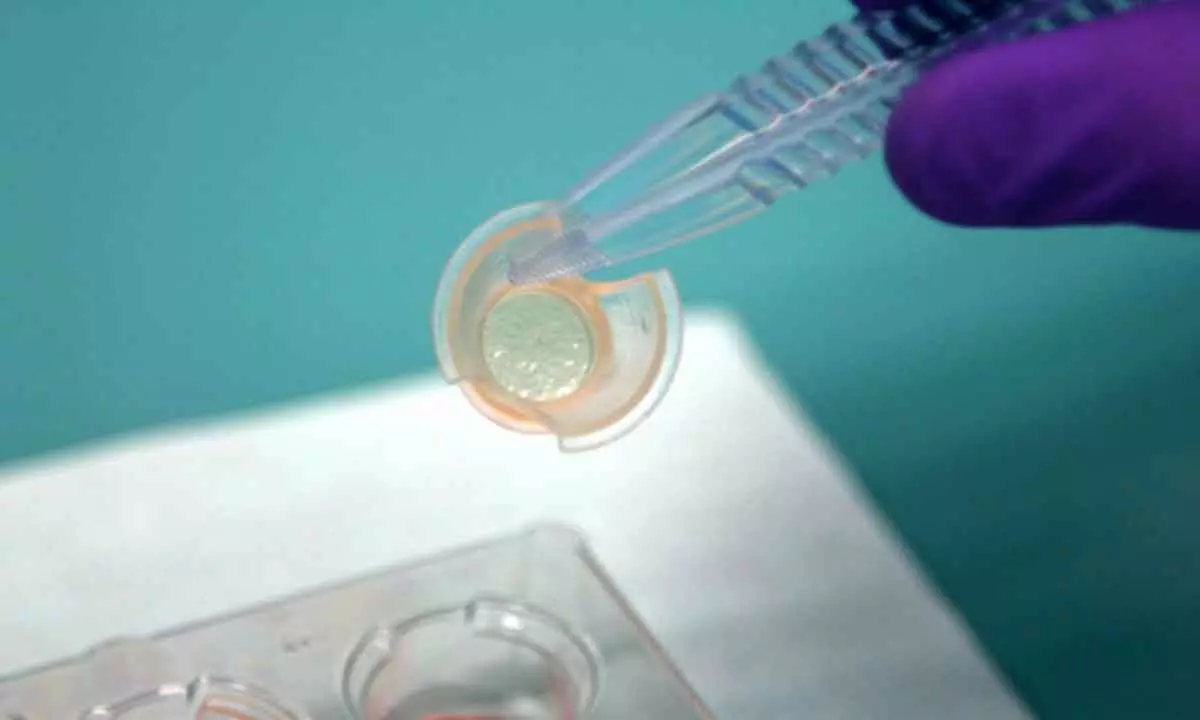
Computer simulations or “in silico models” are one example. These can be used across the medical product development process to complement – and in time potentially replace – other model types. They can be used in drug studies to model a drug’s behaviour within the body, from cellular interactions to processes that involve multiple organs. Complex three-dimensional biological models are also maturing quickly. Examples include: organoids – organ “buds” that can be propagated from stem cells or taken from biopsies organs-on-chips – cells cultured in a miniature engineered chip. These attempt to replicate the physical environment of human organs
Canberra: Animals have been used for medical research for thousands of years, dating back to ancient Greece where the first dissections were performed. These days, one of the main uses of animals is to ensure the safety of medical products before they’re trialled in humans. But in addition to the important ethical reasons for minimising animal use, the reality is sometimes animals just aren’t that good at predicting human responses. No animal model, for example, has captured all the human characteristics of complex illnesses like Alzheimer’s disease or chronic inflammatory demyelinating polyneuropathy (a neuromuscular disease). This makes is hard to develop effective treatments and cures.
Thankfully, researchers are making progress in developing a collection of alternative approaches, called “non-animal models”. A new report from our team at CSIRO Futures examines the potential of non-animal models and the actions Australia will need to take to pursue their use.
Non-animal models
Non-animal models are an alternative set of models that use human cells, tissues and data. These have the potential to better mimic human responses. In doing so, this can more accurately predict if a medical product is likely to fail, allowing reinvestment in products that are more likely to succeed. Computer simulations or “in silico models” are one example. These can be used across the medical product development process to complement – and in time potentially replace – other model types. They can be used in drug studies to model a drug’s behaviour within the body, from cellular interactions to processes that involve multiple organs. Complex three-dimensional biological models are also maturing quickly. Examples include: organoids – organ “buds” that can be propagated from stem cells or taken from biopsies organs-on-chips – cells cultured in a miniature engineered chip. These attempt to replicate the physical environment of human organs.
What can we use these for?
In theory, we can use non-animal models for everything we use animal models for – and more. Simple non-animal models (human cells cultured over a flat surface) are already used to help identify drug targets due to their ability to test a large number of compounds and experimental conditions. In the future, non-animal models will reduce – and eventually replace – animal use across a range of applications: screening potential drugs to see how well they work toxicology (safety) testing helping to screen, select and stratify shortlisted participants for clinical trials.
This might include an assessment of their unique response to a potential drug. using patient cells to identify the treatment most likely to help that individual. Outside of medical products designed for humans, non-animal models can also support innovation in veterinary and agricultural medicines, cosmetic testing and eco-toxicology. An export opportunity for Australia Non-animal models present an economic opportunity for Australia, where the models, their components, and surrounding services could be exported to the world. Our novel economic analysis sized the potential Australian market for two non-animal models: organoids and organs-on-chips. Other models were unable to be sized due to a lack of global market data.
We estimate the Australian organoid market could be worth Australian dollar 1.3 billion annually by 2040 and create 4,200 new jobs. The organs-on-chips market could be worth Australian dollar 300 million annually by 2040 and create 1,000 new jobs. This estimate is lower as this technology is currently less advanced but holds the potential to grow significantly beyond 2040. Several Australian entities are already contributing to these opportunities.
The Murdoch Children’s Research Institute, for example, provides stem cell and modelling expertise as part of reNEW, a €300 million international collaboration. Another example is from Schott Minifab, an international biotech and medical device company with Australian roots, which has successfully established scaled production of non-animal model components in Australia for domestic and export markets.
Making it a reality
Non-animal models have already begun to complement and replace animal use in some areas, such as identifying drug targets. However, accelerating their development and adoption across a wider range of applications will require further technical advances to lower cost and validate their performance as superior models. Australia has several research strengths in this field but we need a concentrated effort to help our research make it through to real world impact. Our report makes ten recommendations for supporting Australia’s pursuit of these opportunities. Critical activities over the next five years include: coordinating local capabilities investing in upgraded infrastructure creating and collating data that compares animal and non-animal model performance. Governments, industry and research must collaborate to deliver against these actions. Success will only come from collective efforts.
(The Conversation)








